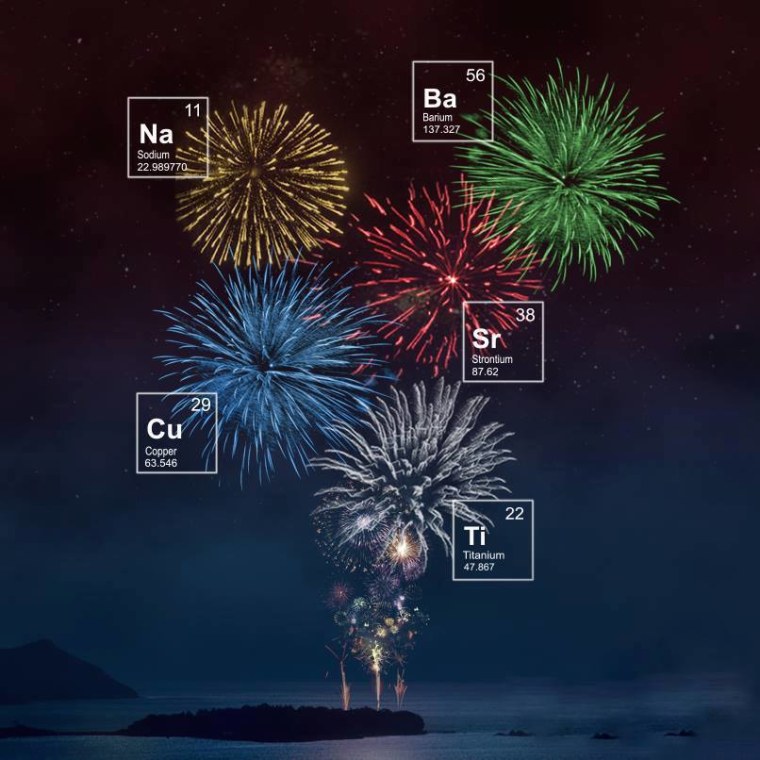
Every wonder how they make all those gorgeous explosions we all enjoyed yesterday? You guessed it -- SCIENCE.
The color you see is a result of the atoms in the fireworks reaching hot enough temperatures that the electrons inside get so excited they give off energy. This energy is emitted as light, or photons, as a result of the electron "falling" from a higher energy level to a lower one in its orbit around the atomic nucleus. The color of the light we see as a result depends on how far that electron "fell." Scientists characterize this distance using wavelength or frequency.
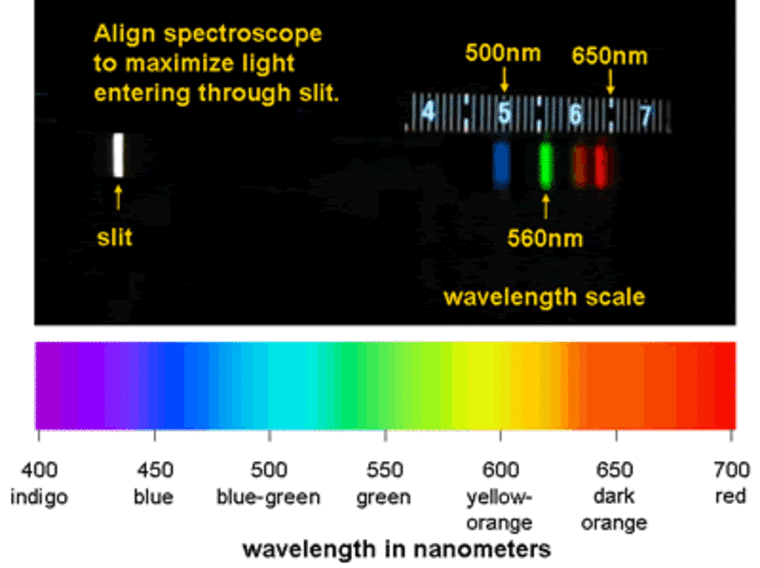
When electrons in sodium atoms get excited enough to emit photons, the resulting light has a wavelength of approximately 590 nanometers ,which is in the yellow part of the visible spectrum -- so fireworks with sodium look yellow to us. Similarly, blue fireworks come from copper, green from barium, and red from strontium. The visible spectrum spans roughly 400 to 700 nanometers, from blue to red, so the atoms in each of these elements give off light with wavelengths corresponding to their part of the spectrum.
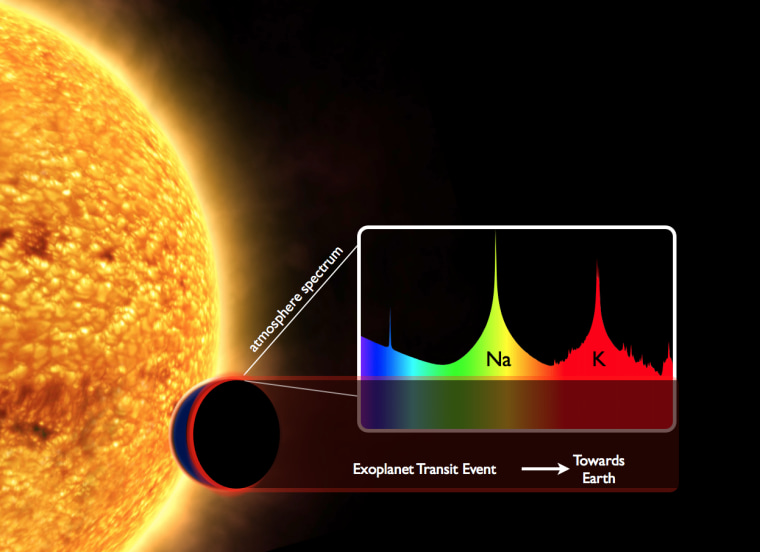
The amazing thing about this process is that it's not only responsible for explosions here on Earth, but it's also what astronomers use to study explosions in the sky. Astronomers can infer what elements a star is made of by studying the colors in the light we collect with telescopes. By using digital cameras and instruments called spectrographs, we can see how much light is coming from a given star at each wavelength. Then, using our knowledge of chemistry and which elements emit (or conversely absorb) photons at each wavelength, we can back out what elements must be present to produce the light we see. Astronomers can do this for stars, planets, and even gas and dust clouds. In fact, spectroscopy is one of the most powerful tools astronomers have for studying the Universe.
Of course, there's a lot more to making fireworks than just getting the colors you want. For more on the actual construction of them, I refer to this great infographic from the Arizona Daily Star. And if you really want to know more, Ethan Siegel goes into detail for you on his aptly named blog: Starts With a Bang.
Here's some more geek from the week:
- Biology students in Denmark discover that starfish can eject tagging microchips from their bodies.
- Scientists are excited to study this massive community of diving tarantulas in Australia's Northern Territory. Wouldn't you be?
- The amazing story of how vanilla plant came to be domesticated.
- Headed to the beach this summer? Here's the chemistry behind that "fresh sea air" smell.
- Clint Eastwood and the leaning tower of Pisa are helping scientists learn about your brain.
- Wearable tech is poised to take over jewelry lines, starting with this necklace that plays movies.
- Can we program robots to be ethical? What does that even mean?
- What do drumming and math have in common? Fractals!
- Hypnotizing 3D visualization of the exoplanet system around Beta Pictoris.
Keep on geeking!
@Summer_Ash, In-house Astrophysicist