
Scientists at Stanford University have solved the mystery of how drops of food coloring appear to dance with each other. What started as mere curiosity has now led to a finding that has widespread benefits across the semiconductor and alternative energy industries.
Manu Prakash, Nate Cira and Adrien Benusiglio are all researchers in the bioengineering department. When Cira was an undergraduate student, he was struck one day by how drops of food coloring slid across the glass side he deposited them on for no apparent reason. This stayed with him all the way through graduate school (at Stanford) where he met Prakash and Benusiglio. They discovered the motion they observed was due to the interactions between the two chemical compounds that make up food coloring: water and propylene glycol. In such a two-component fluid, each compound retains its own molecular identity.

In this case, surface tension and evaporation are the driving forces. Water evaporates faster than propylene glycol and also has a higher surface tension. The different in these two parameters is enough to prevent an even distribution of both compounds within each drop. Water ends up concentrated on the top and propylene glycol on the bottom. In essence, the drops become top heavy, and with enough evaporation, they start to "roll."
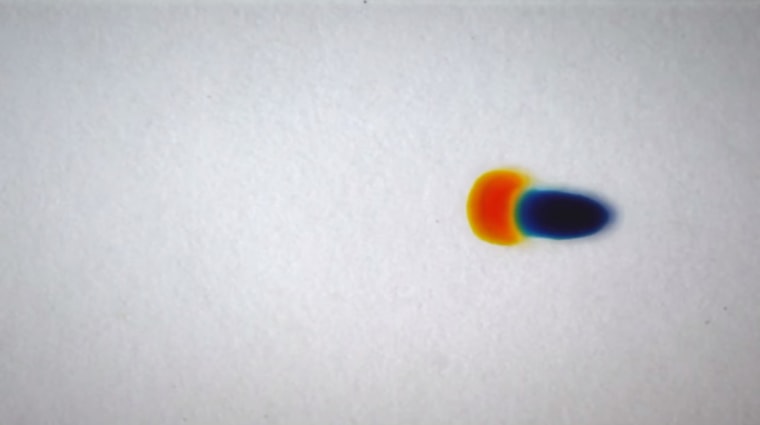
The various dances you see in the video are the result of drops with varying mixtures of water versus propylene glycol that the researched experimented with to see how the motion of the droplets would change. They found they could make drops appear to chase each other and align. It's an amazing glimpse into how complexity can arise from such a simple set-up.
And now for some less rhythmic geek:
- Background music is especially detrimental to older people, not just for hearing but also for memory.
- British engineers are working on building the world's fastest car.
- Last month, Byron Jones broke the standing long jump record. Here's the physics of how he did it.
- Why moving your cell phone a couple inches to the left can actually boost your signal. [VIDEO]
- The Eiffel Tower now doubles as an electrical generator. [VIDEO]
- The pros and cons of 3D printed prosthetics.
- Can a robot paint a masterpiece good enough to fool experts?
- This artist creates elaborate and realistic images of space from stuff he has in his kitchen. [SLIDESHOW]
- NASA has released a desktop app to crowd source asteroid detection.
- The next rover NASA sends to Mars will have an instrument on board to produce oxygen from carbon dioxide in the Martian atmosphere.
- With this past week's solar eclipse and next week's lunar eclipse, check out this crash course on eclipses with Phil Plait. [VIDEO]
Keep on geeking!
@Summer_Ash, In-house Astrophysicist